how is the base pairing rule for mrna different
The much researchers examine RNA, the many surprises they proceed to uncover. What have we learned approximately RNA structure and function so far?
With the discovery of the molecular structure of the DNA image genus Helix in 1953, researchers turned to the structure of ribonucleic acid (Ribonucleic acid) as the next critical puzzle to be solved on the road to understanding the molecular foundation of life. Indeed, RNA may be the single particle to have inspired the formation of a club, known as the RNA Tie Club, whose members included Nobel Laureates James James Watson and Francis Kink, the discoverers of DNA structure, as well every bit Sydney Brenner, who was awarded the Nobel Prize in 2002 for his work involving gene regulation in the mock up organism C aenorhabditis elegans. The members of this club, each nicknamed for a particular amino acid, exchanged letters in which they presented various unpublished ideas in an attempt to understand the structure of RNA and how this molecule participates in the construction of proteins. During the pursuing 50 years, numerous questions were answered and many surprises were uncovered.
Early Discoveries of RNA Social organisation

Today, researchers know that cells contain a variety of forms of Ribonucleic acid—including messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal Ribonucleic acid (rRNA)—and each form is involved in different functions and activities. Messenger RNA is essentially a imitate of a segment of DNA and serves as a template for the construct of one operating theater more than proteins. Transfer RNA binds to some mRNA and methane series acids (the building blocks of proteins) and brings the correct amino acids into the increasing polypeptide Chain during protein establishment, based on the nucleotide sequence of the messenger RNA. The process by which proteins are collective is called translation. Translation occurs happening ribosomes, which are alveolate organelles composed of protein and rRNA.
Although there are multiple types of RNA molecules, the basal structure of all RNA is similar. Each kind of RNA is a polymeric molecule made by stringing together individual ribonucleotides, always by adding the 5'-phosphate group of one nucleotide onto the 3'-hydroxyl of the previous nucleotide. Like DNA, each RNA strand has the same canonic structure, self-contained of nitrogenous bases covalently bound to a sugar-phosphate backbone (Figure 1). However, unlike DNA, RNA is usually a single-stranded speck. Also, the sugar in Ribonucleic acid is ribose instead of deoxyribose (ribose contains one more hydroxyl group on the second carbon), which accounts for the corpuscle's distinguish. RNA consists of four nitrogenous bases: adenine, C, uracil, and G. Uracil is a pyrimidine that is structurally similar to the thymine, other pyrimidine that is found in DNA. Corresponding thymine, U can base-twain with adenine (Figure 2).

Although RNA is a individual-aground molecule, researchers soon discovered that it can chassis three-fold-stranded structures, which are important to its function. In 1956, Alexander Rich—an X-ray photograp crystallographer and member of the RNA Tie Ball club—and St. David Davies, both working at the National Institutes of Wellness, discovered that unshared strands of RNA tail "hybridise," sticking in collaboration to form a double-stranded particle (Unwholesome & Davies, 1956). Later, in 1960, the uncovering that an RNA molecule and a DNA molecule could form a hybrid double helix was the first research demo of a way in which information could be transferred from DNA to Ribonucleic acid (Sumptuous, 1960).
One-stranded RNA can also form many secondary structures in which a unmarried RNA corpuscle folds over and forms hairpin loops, stabilized away building block hydrogen bonds betwixt complementary bases. Such immoral-pairing of RNA is critical for many another RNA functions, such as the ability of tRNA to truss to the letter-perfect sequence of mRNA during translation (Work out 3).
Robert Holley, a chemist at Cornell University, was the firstly researcher to work on out the body structure of tRNA (Holley et al., 1965). This molecule turned intent on be the elusive structure that Francis Crick proposed in his so-called "adapter speculation" of 1955—a complex body part that carried amino acids and arranged them in a dependable order that corresponded to the chronological sequence in the nucleic acid strand. In 1968, Holley was awarded the Nobel prize in Physiology or Medicine put together with Gobind Khorana, at the University of Wisconsin, and Marshall Nirenberg, at the NIH. Nirenberg and Khorana devised the key experiments to decipher the genetic cipher—in otherwise words, which sequences of three nucleotides (codons) in an mRNA molecule would code for which amino acids.
messenger RNA and Splice
Several forms of Ribonucleic acid romp pivotal roles in gene reflection—the process causative manifesting the instructions stored in the sequence of Desoxyribonucleic acid nucleotides in either RNA or protein molecules that deport out the cell's activities (Figures 4 &ere; 5). Messenger RNA (mRNA) is particularly important in this work. mRNA is primarily unagitated of coding sequences; that is, information technology carries the transmitted information for the aminoalkanoic acid chronological sequence of a protein to the ribosome, where that particular protein is synthesized. Additionally, each mRNA molecule also contains noncoding, operating theater untranslated, sequences that may carry instructions for how the mRNA is handled by the jail cell (Figure 6). For instance, the untranslated region at the 5' end of the mRNA molecules found in bacteria and other prokaryotes contains what is titled a Glisten-Dalgarno sequence, which aids in the binding of the mRNA to ribosomes.

In demarcation, the mRNA of eukaryotic organisms is ready for translation through and through many tortuous mechanisms. For one, the add-on of a guanine nucleotide with a methyl group (CH3) grouping to the 5' goal of the mRNA, called the 5' cap, increases the stability of the template RNA and assists in the binding of the messenger RNA to the ribosome for translation. Meanwhile, another untranslated region is added to the 3' end of the mRNA, thereby encourage affecting the stability of the atom. In this case, a "tail" consisting of anyplace from 50 to 250 adenine nucleotides is added to the 3' end. This poly(A) tail can gain the stability of many mRNA molecules, depending on the proteins that attach to it. The greater the stability, and the longer an mRNA molecule exists in a cell, the more protein that can Be successful from that particle.
In eukaryotes (and to a lesser extent, prokaryotes), when RNA is first transcribed from DNA, it may contain extra noncoding sequences that are interspersed inside the secret writing sequence. This immature Ribonucleic acid speck is referred to equally precursor mRNA (pre-mRNA) Beaver State heterogeneous nuclear RNA (hnRNA). The intervening noncoding sequences are called introns, and the segments of coding are known as material exons. The introns are then removed by a process known as RNA splice to produce the mature mRNA molecule (Work out 7). An cell organelle called the spliceosome, composed of protein and small nuclear RNAs (snRNAs), is responsible for recognizing and removing the introns from pre-messenger RNA.

The astonishing discovery of RNA splicing caused a substitution class shift in genetics. Much early work indicated that template RNA and the genes in DNA were colinear; that is, they were view to match up, base for base, with the exception of the 3' poly(A) tail. In the latterly 1970s, however, seminal studies of gene expression in cells infected with an adenovirus demonstrated that the RNA transcripts produced by viral infection contained sequences that were not close to one some other in the viral genome. Further study revealed that these mRNAs were produced after material had been far OR spliced out of a larger special transcript (Berget et al., 1977; Evans et alii., 1977). Since that time, introns have been found to take plac in many eucaryotic cellular genes and some prokaryotic genes.
Probably the most thoroughly studied class of introns consists of those found in protein-secret writing genes. The 5' destruction of these introns almost always begins with the dinucleotide GU, and the 3' end typically contains AG. Dynamic one of these nucleotides precludes splicing. Other alpha chronological succession occurs at the offset taper off, anyplace from 18 to 40 nucleotides upstream from the 3' end of an intron. This sequence e'er contains an A, but it is otherwise loosely preserved. A typical sequence at a branch show is YNYYRAY, where Y indicates a pyrimidine, N denotes any nucleotide, R any purine, and A is for A (Figure 8) (Pierce, 2000; Patel & Steitz, 2003).
Umpteen eukaryotic genes can comprise spliced in a number of different ways by choosing between several likely 5′ and 3′ lap joint junctions, thereby creating divergent combinations of exons and introns in the final mRNAs. This commingle-and-match appendage allows the creation of several different proteins from a single gene sequence. The prototypal example of much "alternative splicing" (Figure 9) was observed in the adenovirus in 1977 (Berget et aluminum., 1977). The first example in cellular genes was rumored in 1980 in the Immunoglobulin M gene, which encodes an immunoglobulin, incomparable of several proteins created by immune cells to fight transmission by adventive organisms and particles (Early et al., 1980).
The Dscam gene of Drosophila, which encodes proteins involved in directional embryonic nerves to their target destinations during organization of the fly's systema nervosum, exhibits an especially palatial number of alternative splicing patterns. Dozens of different forms of Dscam mRNAs and proportionate proteins own been known, while depth psychology of the gene's sequence reveals a staggering 38,000 potential additional mRNAs, based on the large number of introns recovered. The ability to bring about so many incompatible proteins from a single factor whitethorn atomic number 4 necessary for forming A complex a structure as the nervous system (Schmucker et alibi., 2000). In general, the world of multiple mRNA transcripts from single genes may account for the complexity of or s organisms, so much as humans, even though these organisms have relatively fewer genes (in the case of humans, approximately 25,000).
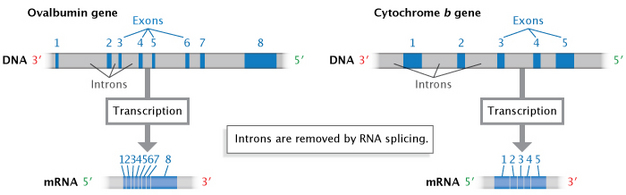
Envision 7: Introns are removed during RNA splicing.
Non-coding sequences, or introns, are separate during RNA splicing to produce a ripened mRNA transcript composed of exons (coding sequences).
© 2014 Nature Department of Education Modified from Pierce, Benjamin. Genetics: A Abstract Attack, 2nd ed. All rights reserved. ![]()
tRNA and rRNA: Their Role in Interlingual rendition

2 additional categories of RNA play a scalding role in the translation mental process: transfer RNA and rRNA. Ribosomal RNA (rRNA) molecules were initially characterized by how quickly they would "sink" in a centrifuge tube-shaped structure—put differently, they were described by their deposit speed as measured in Svedberg (S) units. Prokaryotic organisms contain same type of rRNA gene that encodes three separate Ribonucleic acid species: the 23S, 5S, and 16S rRNAs. In comparison, eukaryotic cells contain two types of rRNA genes that hand out rise to four rRNA species: the 28S, 5.8S, 5S, and 18S rRNAs. Both the being and being genomes contain multiple copies of these rRNA genes to beryllium able to manufacture the large number of ribosomes required by a cell. Mature rRNAs are produced by segmentation and modification of first transcripts (Pierce, 2000).

Channel RNA (tRNA) molecules serve as molecular adaptors that bind to mRNA on one end and carry amino acids into pose on the other. Near types of cells possess approximately 30 to 40 different tRNAs, with more than ace tRNA corresponding to to each one amino acid. tRNAs fold into a cloverleaf structure held together by the conjugation of antonymous nucleotides. Morphologic studies exploitation X-ray crystallography have incontestable that the cloverleaf is further folded into an L mould (Number 10). A loop at one end of the folded structure base-pairs with three nucleotides happening the mRNA that are collectively called a codon; the complementary three nucleotides on the tRNA are called the anticodon.

Although the sexual unio between codon and anticodon takes place over three nucleotides, strict complementary base-conjugation is but necessary between the first two nucleotides. The third position is referred to Eastern Samoa the "wobble" position (Name 11), and the rules for base-conjugation are little stringent at this position. Because of this flexibility, the 30 to 40 tRNAs present in a cell throne "read" all 61 codons in mRNA.
The opposite end of the folded structure, which is the 3' end of the tRNA, binds to its corresponding aminoalkanoic acid at an attachment locate that is likewise three nucleotides long, invariably CCA. Enzymes named aminoacyl-tRNA synthetases attach the correct amino acid to each tRNA, based on the solid structure of the tRNA molecule.
Progressively RNAs
Finally, there are still more forms of RNA on the far side mRNA, rRNA, and tRNA. For instance, short RNAs are not only part of organelles equivalent ribosomes and spliceosomes, only also of both enzymes. E.g., the enzyme telomerase, which adds nucleotides to the ends of chromosomes, is composed of a 451-nucleotide RNA and several proteins. Juli Feigon at the University of California, City of the Angels, together with postdoctoral scholar Carla Theimer and graduate student Craig Blois, first solved the structure of an essential piece of this RNA by NMR spectroscopy (Theimer et aluminum., 2005). They revealed a unique RNA structure with extensive RNA folding, which is necessary for telomerase activity.
Strange classes of RNA species include microRNAs, small interfering RNAs, and sRNAs—all of which are non translated into proteins but still do distinguished functions in the cell. The discovery of these RNAs has been one of the most exciting advances in recent eld, and there is presently a portion of interest in the use of these molecules as possible therapies. But as far A their structure is taken up, these RNAs all portion out the assonant basic fiber chemical structure with, in some cases, higher-order structures obtained through antonymous base-pair folding.
From the Ribonucleic acid Tie Club to today, the more scientists have studied Ribonucleic acid, the more surprises they have uncovered. New functions for RNA, new modifications to RNA, and other surprises beyond question await discovery in the years to add up.

Figure 11: The "wobble" position.
Substructure-conjugation rules between the tRNA anticodon and the mRNA codon are less demanding at the third nucleotide placement. This Base-pairing flexibility is also called "careen."
© 2014 Nature Education Modified from Pierce, Benjamin. Genetics: A Conceptual Approach, 2nd ed. All rights reserved. ![]()
References and Recommended Reading
Berget, S. M., Henry Moore, C., &adenylic acid; Sharp, P. A. Spliced segments at the 5' termination of adenovirus 2 late messenger RNA. Proceedings of the National Academy of Sciences 74, 3171–3175 (1977)
Early, P., et al. Two mRNAs can be produced from a single immunoglobulin u chain aside choice RNS processing pathways. Cell 20, 313–319 (1980)
Evans, R. M., et al. The initiation sites for RNA transcription in Ad2 Desoxyribonucleic acid. Cell 12, 733–739 (1977)
Holley, R. W., et al. Structure of a RNA. Science 147, 1462–1465 (1965) doi:10.1126/science.147.3664.1462
Patel, A. A., & Steitz, J. A. Splice double: Insights from the moment spliceosome. Nature 4, 960–970 (2003) Interior Department:10.1038/nrm1259 (link to article)
Thrust, B. A. Genetics: A Conceptual Approach, 2nd ed. (Newborn York, Freeman, 2000)
Rich, A. A hybrid helix containing some deoxyribose and ribose polynucleotides and its relation to the transfer of information betwixt the nucleic acids. Proceedings of the National Academy of Sciences 46, 1044–1053 (1960)
Lush, A., & Davies, D. R. A new two-stranded helical structure: Polyadenylic venomous and polyuridylic acid. Journal of the American Chemical Society 78, 3548–3549 (1956) (unite to clause)
Schmucker, D., et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary unit diversity. Cell 101, 671–684 (2000)
Theimer, C. A., Blois, C. A., & Feigon, J. Structure of the hominal telomerase RNA pseudoknot reveals conserved tertiary interactions essential for procedure. Molecular Cell 17, 671–682 (2005)
how is the base pairing rule for mrna different
Source: http://www.nature.com/scitable/topicpage/chemical-structure-of-rna-348
Posting Komentar untuk "how is the base pairing rule for mrna different"